
Biologists have long recognized that all animals have ‘normal’ life programmed to live. Elephants typically live 70 years, for example, while fruit flies live only about 30 days.
The lifespan cap for any species is called its Hayflick Limit, named after microbiologist Leonard Hayflick. In the early 1960s, Hayflick animal body) are programmed to divide a certain number of times, then die. This natural cycle determines your lifespan.
According to the Hayfl ick Limit, humans should be able to reach 120 years of age (the same number the Bible says we are supposed to live, interestingly enough). The fact that most of us don’t make it is because we live and eat poorly, and are exposed to excessive toxins, carcinogens and stress.
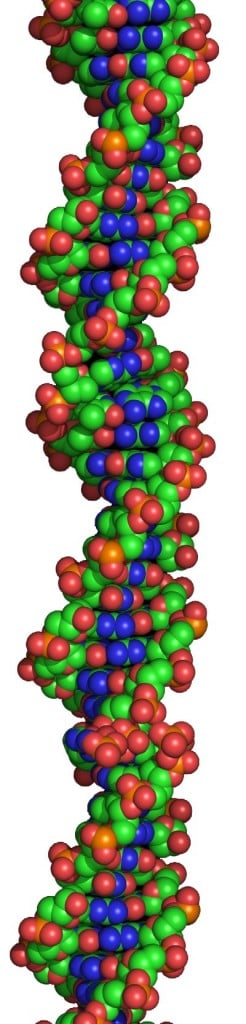
What determines the Hayflick Limit for us humans is something called a “telomere,” which is the cap that holds the DNA strands together in our cells. The telomere acts something like the tip on a shoelace. Each time your cells reproduce, the telomere gets shorter. When it gets too short, the DNA strands fall apart and the cell dies.
What’s exciting about all this is that doctors can now measure the lengths of telomeres, and therefore fi gure out how much time our cells have left—in other words, how much we have aged.
“It’s a pretty unique service,” says Juan Remos, M.D., an anti-aging specialist at The Wellness Institute of the Americas. “We can tell people how long their telomeres are
compared to other people their age.”
That is not exactly the same thing as giving you a definitive biological age, which Dr. Remos says is not possible without testing the telomeres of each organ in your body. “Your brain and your spleen can be diff erent ages,” he says. “We could look at 100 telomeres, and not tell a person’s precise age.” What Dr. Remos tests is the telomere length of of how fast you are aging overall—and which are easier to access than, say, liver cells you’d have to remove in order to test.
The length of your telomere is measured by an electron microscope—Dr. Remos uses a Texas-based lab—and then compared to the length of telomeres from a national database. “If your telomere is in the 98th percentile, it means your telomere is longer than 98 percent of the people darn good shape.
But what happens when your telomeres are short?
First, it means that you are aging rapidly, and that illnesses such as cancer and heart disease. Next, it means you should change your lifestyle to include a better diet, more exercise, more sleep, and lots of quality vitamins and anti-oxidant supplements—‘basically anything that helps repair your DNA,” says Dr. Remos.
Indeed, researchers have already shown that a healthy lifestyle can lengthen your telomeres. In one study conducted in Germany in 2009, a group of serious middle-aged runners (average age of 51, length in their white blood cells. These were compared to other middle-aged but sedentary Germans. The conclusion: Telomere length was 40 percent shorter in the sedentary subjects. Exercise, noted study author Dr. Christian Werner, “at the molecular level has an anti-aging effect.”
So what’s a person to do? Beyond a healthy diet, proper sleep, lots of exercise and a regimen of anti-oxidant supplements, the ultimate solution may be an enzyme called telomerase, which can be produced by genes in our cells and is able to restore telomere length. Dr. Werner reported that his older runners had more telomerase, and he speculated that exercise might affect telomerase activity rather than telomeres themselves.
It will be years before scientists solve this riddle, though they’re already at work on the problem. “[Producing] telomerase is the ideal,” says Dr. Remos. “Already there are companies pursuing this, to prevent telomeres from shortening.” One firm is TA Sciences, a New York-based company that markets a molecule called TA-65, which causes cells to produce telomerase. TA-65 is extracted from astragalus, an ancient herb used in Chinese medicine for more than 2,000 years.
“It’s not telomerase, which exists naturally in the cell,” says Dean Miller, vice president of TA Sciences. “The molecule has a way to turn on the telomerase gene, so it will release the telomerase in the cell.” The herb is expensive—about $200 a month—because it takes five to ten gallons of astragalus to produce one capsule.
So far, the effects of TA-65 are anecdotal, but clinical trials are underway to prove its effectiveness. But the fact that telomerase works was vindicated by three American scientists who received a 2009 Nobel Prize in medicine and physiology for proving its power.
Still, is this the path to immortality, or just to living out our full years? “The human cell has a cycle, and I’m not sure we can alter it,” says Dr. Remos. “In the meantime, you can see if you are relatively younger than most people your age.”










